Nothing can hold out against civilization and the power of industry. The only animal species to survive will be those that industry multiplies.
—Jean-Baptiste Say1
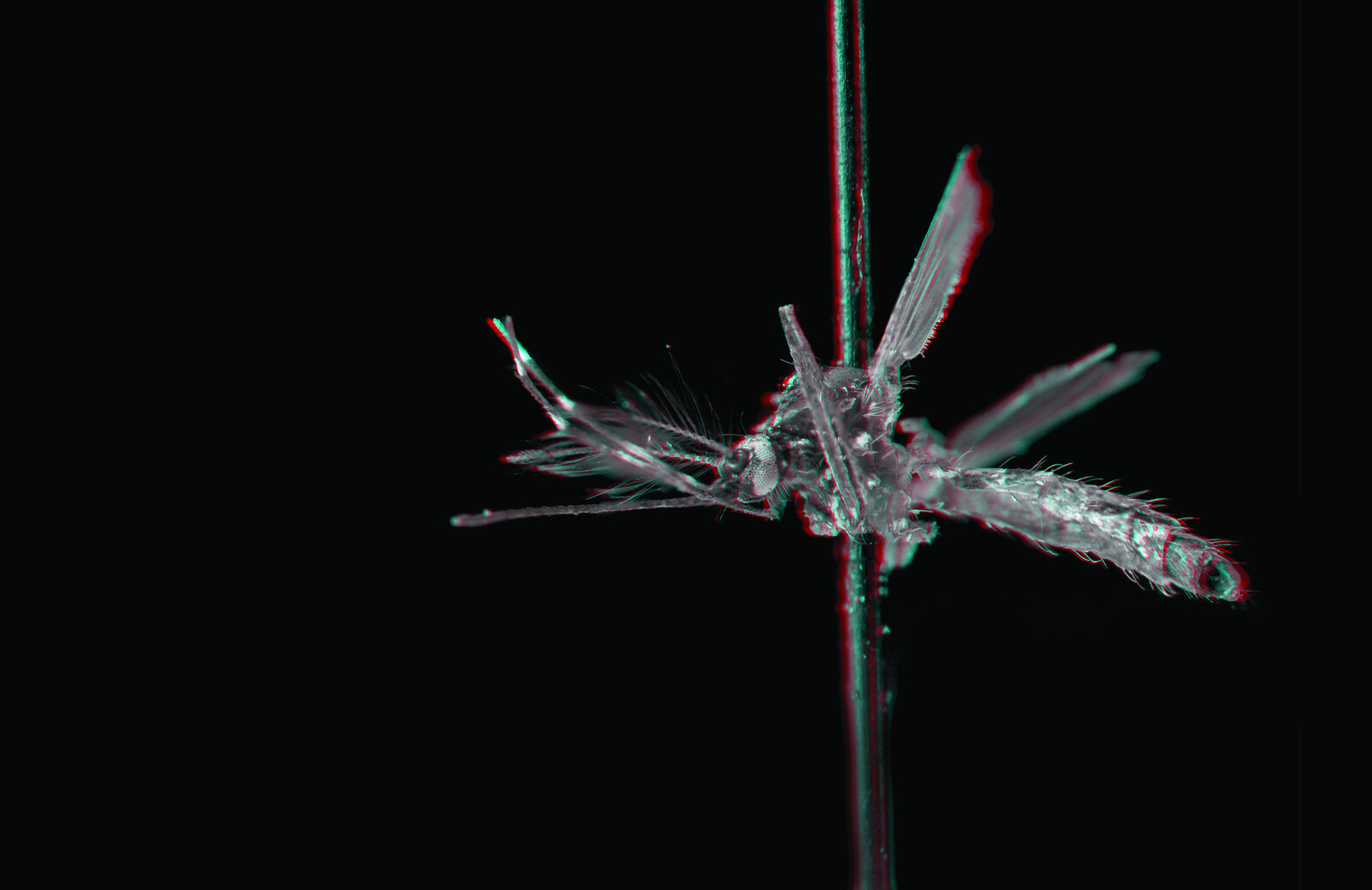
A female Aedes aegypti remains in suspended pregnancy until she ingests vertebrate blood. With hundreds of eggs in her ovaries, she begins a search for carbon dioxide and heat. Once detected, she lands on her host to penetrate the epidermis with her proboscis and deposit saliva, which as an anti-coagulant, ensures her meal of blood will flow smoothly to the next generation. Within sixty hours of this fluid exchange—spit for blood—oviposition is triggered in the expectant Aedes aegypti and her eggs are released along the surface line of still water where they complete their embryogenesis and wait. As rainwater delivers new microorganisms into this watery exometabolic womb (so often unwittingly prepared by humans), respiration reduces the available oxygen and causes the eggs to hatch. Without vows or affection, humans and mosquitoes become kin, bound by blood to the rhythm of microbial breath.
Mosquitoes sucked the blood of vertebrates for millions of years before Homo sapiens emerged from the evolutionary phylum, and until recently, humans weren’t particularly appealing hosts. But then they tilled, irrigated and settled, and as the Sahara dried, their settlements became the primary source for the blood and water that Aedes aegypti needed to survive. A sylvan arthropoid first domesticated the land, and was in turn domesticated as host; an evolutionary lesson in becoming-hospitable.2
While settlement patterns drew almost all of Aedes aegypti’s host species near—dogs, cats, rodents, cows, pigs, and birds—they still came to favor blood from its human architects. Due to low levels of isoleucine, the blood of Homo sapiens extends the life of Aedes aegypti and ensures the production of offspring counts in the thousands. Not only that, but with higher levels of lipids than in other primates, human blood helps co-produce thirstier vectors.3 Because mosquitoes’ sanguine preference is based on their first successful blood-feed, as humans become ever more densely arranged into their urban settlements, the likelihood that Aedes aegypti’s first feed will be on human blood increases in turn, thereby establishing a lifelong affection; affine commitments are this way produced without the awkward requirement of affinity.
As humans continue to design and deploy new techniques intended to sever ties with this unwanted bloodline, the fact that mosquitoes rapidly overcome the best efforts to keep human blood sacred should come as no surprise. For Aedes aegypti, it is precisely those terrestrial, aquatic, and atmospheric architectures designed to deny them any access to Homo sapiens’s blood that have proven to be the most compelling invitation to intensify this multispecies co-evolution.4
The Virus
Individual animals and plants are like temporary “experiments” with which gene pools probe current environmental conditions to make sure that past successes are still viable.
—Manuel DeLanda5
When Aedes aegypti takes blood from a viremic host in the early stages of dengue, the blood moves into the mosquito’s midgut, where the virus binds to receptors, enters the circulatory system and makes its way to the salivary glands. Once sufficient viral replication has occurred in the glands—a process lasting four to ten days on the calendar adopted by most Homo sapiens—the virus is transmissible to other humans. The mosquito remains infected for the rest of its life.6
When the dengue virus quietly initiated this “vectors-with-benefits” relationship, the Aedes aegypti was its sole host; then, as the virus evolved to live in primates, they became a preferred an agent of transmission. During each infection, dengue renovates the genetic structure of its host by altering no fewer than 147 proteins in Aedes aegypti’s RNA, thereby repatterning the mosquito’s behavior. In essence, the virus makes the mosquito over as an ideal vector: smelling more acutely and hungrier for human blood, infected Aedes aegypti are also more likely to re-feed after interruption (when swatted at, for example, by a human). The dengue virus also uses the RNA alteration as a means to adjust mosquitoes’ saliva production, making it even more hospitable to the virus.7
When an infected mosquito follows its heightened senses to feed on human blood, virus-rich saliva is deposited beneath the epidermis at the start of the meal. In fact, the virus is likely to be passed subdermally several times as the Aedes aegypti is a skittish feeder. Once inside the human body, the dengue virus quickly spills over from the saliva to the epidermal cells and then on to the lymph nodes, where it spreads throughout the lymphatic system. Three to eighteen days of incubation later, symptoms emerge. But, before they do—typically a day or two before the sudden onset of fever—the virus is already transmissible. Homo sapiens hosts are not simply unwitting victims of the Dengue virus received from mosquitoes, they are also unknowing perpetrators who, while making multispecies kin, transmit the virus the the next generation to Aedes aegypti.
The dengue virus has itself been adapting to the changing behavior of Aedes aegypti for thousands of years.8 As these mosquitoes migrated across the taxonomic register from zoophagous to anthropophagus, dengue adapted to live better among humans as well, ensuring the largest spatial range through its host. Dengue can also infect the same human host four times, with each illness more painful and life-threatening than the previous one; because human antibodies cannot situate their relationship to a similar-but-distinct dengue serotype, they tend to boost, rather than prevent, the sickening effect of any novel strands of dengue. Human weaknesses such as these are not left unexploited in the arms race of adaptation. This adaptive dynamism of the virus has been the most consistent obstacle to the creation of a vaccine, several of which have been in development for nearly 100 years.9 Likewise, pathologists are concerned that, given the virus’s highly mutable and adaptable character, it would even be able to adapt advantageously to anthropogenetically modified vectors.10
The Vector
With tears and toiling breath,
I find thy cunning seeds,
O million-murdering Death.
I know this little thing
A myriad men will save.
O Death, where is thy sting?
Thy victory, O Grave?
—Ronald Ross11
For most of the nineteenth century, dirt, debris, and immorality were usually cited as causes of malaria. Then, in 1897, Ronald Ross, a young British scientist, cultured twenty mosquitoes from larvae collected in Secunderabad, India, and paid his patient Husein Khan eight annas to let the mosquitoes feed on his malarial blood.12 One month later, he dissected these mosquitoes to confirm the presence of the malaria parasite in the blood of their midgut.13 By 1902, the Syrian scientist H. Graham also found that the dengue virus was transmitted by mosquitoes, writing: “Besides its maleficent function as the transmitter of malaria and yellow fever and its general character as pestilent nuisance, there is yet another disease of tropical and warmer temperate regions that is being credited to its mischief: Dengue.”14 These discoveries immediately and radically redesigned public health efforts around the world to focus on the eradication of the vector.
In his remarkable study of atmoterrorism, philosopher Peter Sloterdijk has noted that the characterization of pestilential life—in his example, rodents as vermin—led to a particular disposition toward exterminism as a solution to problems of human settlement. He is careful to explain this comportment, noting: “Exterminism constitutes a simplification of sadism as classically described by [Jean-Paul] Sartre. No longer a mere question of usurping the other’s freedom, it is primarily concerned with freeing one’s own freedom from the freedom of others.”15 Most urgent for any consideration of so-called vector-borne illnesses such as dengue is not, despite its decisive political consequences, the ability for scientists to transfer their enthusiasm for exterminism from one species to another, but rather that the exterminist has as his objective the elimination of the environmental conditions upon which his victims’ lives depend. For Sloterdijk, prior to the development and combat deployment of chlorine gas in Ypres, Belgium by the German army during the spring of 1915, humans could more or less assume that the atmosphere upon which they depended was a stable source of life; but by introducing techniques of warfare into the environment itself, it is rendered (or “explicated,” in Sloterdijk’s terminology) as an existential medium that cannot be taken for granted. Because of this, “The twentieth century will be remembered as the age whose essential thought consisted in targeting no longer the body, but the enemy’s environment.”16 Indeed, this is also the case for human settlements in the tropics, where the discovery of the Aedes aegypti as a vector also led to a new exterminist imaginary.
“If an enemy’s body can no longer be liquidated with direct hits,” Sloterdijk contends, “the attacker is forced to make his continued existence impossible by his direct immersion in an unlivable milieu for a sufficiently long period of time.”17 As public health officials came to better understand the life cycle of the dengue virus, the war changed its front; Aedes aegypti were no longer just irritating pests, they were harboring a formidable enemy. Mosquitoes became vectors; agents capable of transmitting infectious pathogens to other organisms, including humans. In response, not long after WWII one begins to find among the budgets of municipal administrators in Southeast Asia a new line item: “Fogging.” Thus began the interspecies war of atmoterrorist eradication that has become so banal that few urban residents bother to give it second thought, even as clouds of toxic fog are repeatedly sprayed into their homes, mosques, and schools.
Nonhuman Settlements
Getting hungry, eating, partially digesting, partially assimilating, and partially transforming: these are the actions of companion species.
—Donna Haraway18
For thousands of years, as humans re-designed other animals in order to improve their utility and obedience through breeding and domestication, third-tier synanthropes were co-domesticating alongside them. On 25 May, 1779, David Bylon, a Dutch surgeon living in the Dutch colonial city of Batavia (now Indonesia’s capital, Jakarta), suddenly became ill with a severe fever, causing him to leave the company of his companions and go to bed early. In what is cited as the first clinical report of Dengue from the torrid zone, Bylon describes intense muscle and joint pains into the third week of his illness. Ultimately, he fully recovered, yet Bylon’s illness represents one case among an ongoing and often devastating cycle of epidemics reported in Asia, Africa, and North America throughout the 1780s, all of which followed the broader trend of outbreak patterns occurring along colonial shipping and trade routes. Initially moving from Africa to the Americas aboard slave ships, the range of the dengue virus was expanded while Aedes aegypti were fervently breeding among the countless casks of stagnant water accompanying colonial troops.
Dengue broadened its geographic reach again by travelling aboard military planes and boats during WWII, initiating the emergence of distinct viral genotypes. The post-war economic boom also resulted in heightened international trade, and the movement of both goods and people were accompanied by the Aedes aegypti, further distributing the virus. In 1953, a child in Manila became the first patient to die from dengue. By this point, the virus had manifested into more severe forms, including dengue hemorrhagic fever and dengue shock syndrome, which cause a combination of bleeding from the nose, ears, or underneath the skin, vomiting, and circulatory shock caused by plasma leakage into the interstitial spaces of the body. Inevitably, the increasing severity of symptoms caused the war of extermination to intensify in Southeast Asia.
By 1968, numerous cases of dengue were reported in Indonesia. By the end of the year, fifty-seven clinical cases and twenty-four deaths were reported and a national public health campaign to identify and treat dengue cases was initiated. The following year, fogging technologies were deployed for the first time in Jakarta. Their purpose was as simple as their design: spray organophosphate pesticides into the air to kill Aedes aegypti populations. It is now known that dengue’s RNA structure produces one mutation every genome replication, largely because it does not go through a “proof-reading” stage, resulting in the pathogen being genetically destined for expansion and diversity.19 Four genetically distinct serotypes and nearly fifty genotypes have developed in the last three hundred years, thus guaranteeing its survival in distinct geographic niches and among various demographics.20 Today, dengue is endemic in over 100 countries, with 400 million people a year becoming ill from the virus; it is thirty times more common than the flu and hospitalizes half a million people annually.21
While the domestication of nonhuman species contributed to the development of denser human settlements, the designs that guided this manipulation of genetic development also created the conditions for pathogens to reproduce, mutate, spread, and find a preferable host species in Homo sapiens. Dengue’s genetic typos are designed for urban living, with diverse genetic pools offering niches for experimental virus strains to survive. The urban landscapes of human settlements were thus optimal environments for the dengue virus to re-design itself through expeditious reproductive cycles, while also strengthening the fitness of its preferred vector—Aedes aegypti.


Left: In an effort to remove a tree stump from the pathway of a planned drainage ditch, the malaria control health worker uses an auger to dig a hole for dynamite sticks. From the 1920s to the 1950s, the use of explosives was widespread in the southern United States as part of the effort to eradicate standing water bodies that hosted malaria vectors. Courtesy of the CDC. Right: A team of malaria control health workers pose for a photograph as they prepare to dig, detonate, and remove a tree stump from the path of a planned drainage ditch. Courtesy of the CDC.
The Fog of War Machines
Governance is well named. It describes well the destruction of what is implied by a collective responsibility with regard to the future, that is to say, politics.
—Isabelle Stengers22
If you ask anyone in Jakarta why groups of men are stalking narrow residential streets with awkward silver boxes slung around their sweat-soaked bodies, it is likely they will reply that the fog being dispersed from their machines kills mosquitoes. This is only partially true. These war machines mix gasoline and pesticide in a heated chamber designed to produce microscopic droplets of insecticide; the machine’s proboscis then ejects a thick, white cloud, dramatic and eruptive. The “fog” is directed toward the most common places in the Aedes aegypti’s environment, usually dark nooks, hidden corners, and covered ditches. Yet, the target of the fog is only nominally the Aedes aegypti. More precisely, the fog of these war machines is intended to make the environment within which dengue lives unlivable. Although Homo sapiens are no less present in this host “environment,” the “fog” is not meant to kill them, at least not immediately; death is a matter of latency.


A fumigation team prepares to board cargo ships in the Port of Jakarta, Indonesia, 1969. Courtesy of the CDC Division of Vector-Borne Diseases.
After nearly twenty years of fogging in Jakarta, a national spike of dengue incidences in the late 1980s shifted the deployment of these atmotechnics from being used responsively—where cases were recorded—to a broader, preventative performance. In other words, the war of eradication became total. Subdistrict health officials advised residents to open their doors but vacate their houses for three hours while fogging was taking place and to cover anything that might later come into immediate contact with human skin. Pesticides enter mosquitoes through their outermost organ, the cuticle; cholinesterase then binds to internal receptors which would normally receive the signal to move, paralyzing them completely. The men who carry out the fogging were thus advised to regularly check the level of cholinesterase in their blood to ensure the paralyzing effect was not also bioaccumulating in their own bodies.23 Wars of attrition are not won without sacrifices.
Yet as the exterminism continued, it was not the price paid by frontline human subjects that was most disturbing. Nearly three decades after the first fog of war was deployed against Aedes aegypti in Indonesia, scientists observed among samples of dead mosquitoes something unexpected: the pesticides were no longer working. The studies revealed Aedes aegypti’s resistance to organophosphates, the active ingredient in larvicide and malathion-based fogging.24 Human health agencies responded to these genetic defenses hermeneutically; they re-interpreted provincial law in Indonesia which required officials to “use pesticide rationally” as mandating a rotation of pesticides once every three years, or every three to six months, or to continually increase the dosage of the pesticide.25 Pesticide rotation proved incapable of keeping pace with the twenty-one-day mosquito lifecycle, and dengue, replicating as it does with every new host, produced countless new generations with potentially advantageous mutations.
What began as genetic anomalies in Aedes aegypti became adaptive features guaranteeing the efflorescence of future generations. In Indonesia, Aedes aegypti have doubled their rate of “knockdown resistance genes” in just the past ten years,26 and pyrethroid fog no longer causes paralysis or death.27 Another trajectory of resistance can be found in the 1016G mutation, which produces detoxification enzymes that metabolize pyrethroids before they reach the target site to paralyze the mosquito. Humans are, it should be noted, far from the mosquito’s first adversary; Aedes aegypti already evolved detoxification mechanisms thousands of years ago to cope with the organic chemicals that leached from rotting plant material in the stagnant water of their larval habitats. What is novel about the role of humans in the last century is their ability to design an environment optimized for their mutation and evolution. In 1946, there were only twelve cases of insecticide resistance reported worldwide; in 1990, five hundred species of mosquitoes were resistant to at least one pesticide.28 The human designs for settlement and its atmoterroristic defense became indistinguishable from the environmental pressures within which Aedes aegypti learn to adapt and thrive.29
Pestilential Urbanism
Terrorism is the maximal explication of the other from the point of view of his exterminability.
—Peter Sloterdijk30
After nearly fifty years of intense pesticide usage (and tens of thousands of generations of mosquitoes), today fogging is synonymous with dengue control in Jakarta. This intangible infrastructure has also gained a monopoly on the human imaginary with respect to multispecies interactions. Despite the limited effectiveness of fogging, the psychosocial comfort it provides for urban residents is significant. Although nearly all public health officials agree that fogging can only be effective when other social behaviors are routinized (such as restricting Aedes aegypti breeding grounds by eliminating stagnant water), simple citywide strategies like dumping or covering water containers have done little to encourage human participation. Fogging is a more demonstrative mode of domination, even if it is less effective, impossible to maintain in the long term, and tends to diminish the importance of behavior-oriented Dengue control efforts.31 Importantly, the amtotechnical performance and compelling visual theatricality of urban fogging still communicates to residents that someone, somewhere is thoroughly governing the environment upon which their health depends.32


A field technician looks for Aedes aegypti larvae in standing water containers during a coordinated eradication program in Miami, Florida, 1965. Courtesy of the CDC.
As with political economies, toxins accumulate differentially among the “respiratory economies” of human agents.33 While Aedes aegypti have evolved to produce detoxifying enzymes, primates like Homo sapiens do not adapt so quickly. The long-term effects of residing among these increasing levels of toxicity are rarely the subject of political debate or independent scientific research.34 More common is the assumption that someone, somewhere, must have rationally weighed out the trade-offs before further perpetuating this interspecies Jus ad bellum. That such wanton hostilities are enabled by an anthropocentric bias is no secret, but the act of standing down as a species also means overcoming the fog of war. As Beatriz Colomina and Mark Wigley have recently suggested, “Design is a defense of the human, but what is being defended is never clear.”35 This ambiguity is perhaps nowhere more disorienting than among the intangible infrastructures of atmospheric governance, which simultaneously enable human settlements at the scale of a tropical megacity and redesign human vulnerabilities in the adaptive arms race of evolution.
Jean-Baptiste Say, Cours complet d’économie politique (Paris: Guillaume, 1828).
Jeffrey R. Powell and Walter J. Tabachnick, “History of Domestication and Spread of Aedes Aegypti: A Review,” in Memórios do Instituto Oswaldo Cruz 108 (2013): 11–17.
L.C. Harrington, J.D. Edman, and T.W. Scott, “Why Do Female Aedes Aegypti (Diptera: Culicidae) Feed Preferentially and Frequently on Human Blood?” Journal of Medical Entomology 38 (2001): 411–422.
Jim Robbins, “How Forest Loss is Leading to a Rise in Human Disease,” Yale Envirionment 360 (23 February, 2016), ➝.
Manuel DeLanda, “Inorganic Life,” Zone 6: Incorporations, eds. Jonathan Crary and Sanford Kwinter (New York: Zone Books, 1992): 149–150.
World Health Organization, “Dengue and Severe Dengue,” ➝.
S. Sim, J.L. Ramirez, and G. Dimopoulos, “Dengue Virus Infection of the Aedes Aegypti Salivary Gland and Chemosensory Aparatus Induces Genes That Modulate Infection and Blood-feeding Behavior,” PLoS Pathogens 8 (2012).
Ibid., Powell and Tabachnick.
The first dengue vaccine, Dengvaxia, was approved by the WHO in April 2016. As of August 2016, Mexico, Brazil, The Philippines, and El Salvador also licensed the vaccine. The cost and a concern that the vaccine will not protect against all strains of the virus have caused many of the 128 dengue-endemic countries to abstain from vaccine purchasing and implementation.
Adrienne Lafrance, “Genetically Modified Mosquitoes: What Could Possibly Go Wrong?” The Atlantic (26 April 2016), ➝.
This poem was written by Ross the night he first discovered that mosquitoes transmit malaria.
Eight annas have a value of less than one US cent.
Robert E. Sinden, “Malaria, Mosquitoes, and the Legacy of Ronald Ross,” Bulletin of the World Health Organization Volume 85, Number 11 (November, 2007): 821–900, ➝.
H. Graham, “Researches on Dengue,” Journal of the American Medical Association 41 (1903).
Peter Sloterdijk, Terror from the Air (Los Angeles: Semiotext(e), 2009), 28.
Ibid., 14.
Ibid., 16.
Donna J. Haraway, Staying with the Trouble: Making Kin in the Chthulucene (Durham: Duke University Press, 2016), 61.
Scott C. Miller and Nikos Vasilakis, “Molecular Evolution of Dengue Viruses: Contributions of Phylogenetics to Understanding the History and Epidemiology of the Preeminent Arboviral Disease,” Infection, Genetics, and Evolution 9 (2009): 157–170.
S.S. Twiddy, E.C. Holmes, and A. Rambout, “Inferring the Rate and Time Scale of Dengue Virus Evolution,” Molecular Biology and Evolution Journal 20 (2003): 122-129.
World Health Organization, “Dengue and Severe Dengue” (July 2016), ➝.
Isabelle Stengers, In Catastrophic Times: Resisting the Coming Barbarism (London: Open Humanities Press / Meson Press, 2015), 54.
Pyrethroids have been the pesticide of choice for nearly twenty years in Indonesia, replacing organophosphates, which were shown to have negative human and environmental health impacts after thirty years of use. The discovery of DDT’s endocrine disrupting effects and subsequent ban initiated the development of pyrethroids, which are heralded by pesticide companies and government alike as the safer options for non-mosquito beings (despite their acute toxicity to fish, bees, dragonflies, cats, and sometimes dogs), while still offering a mortal mosquito knock-down. See Iqbal R.F. Elyazar, Simon I. Hay, and J. Kevin Baird, “Malaria Distribution, Prevalence, Drug Resistance and Control in Indonesia,” Journal of Advanced Parasitology 74 (2011): 41–175.
See Damar Tri Boewono, “Susceptibility of Dengue Haemorrhagic Fever Vector (Aedes Aegypti) Against Organophosphate Insecticides (Malathion and Temephos) in Some Districts of Yogyakarta and Central Java Provinces,” Bulletin Penelitian Kesehatan 35 (2007); and, Kris Cahyo Mulyatno, Atsushi Yamanaka, and Eiji Konishi Ngadino, “Resistance of Aedes Aegypti (L.) Larvae to Temephos in Surabaya, Indonesia,” Southeast Asian Journal of Tropical Medicine and Public Health 43 (2012): 29–33.
Jakarta Provincial Law No 6/2007, “Dengue Hemorrhagic Fever Control.”
Juli Rochmijati Wulandari, Siu Fai Lee, Vanessa Linley White, Warsito Tantowijoyo, Ary Anthony Hoffman, and Nancy Margaret Endersby-Harshman, “Association Between Three Mutations, F1565C, V1023G, S996P, in the Voltage-Sensitive Sodium Channel Gene and Knockdown Resistance in Aedes Aegypti from Yogyakarta, Indonesia,” Insects 6 (2015): 658–685.
Aedes aegypti had a head start building resistance from decades of exposure to DDT, which targets the same channel. Mosquitoes were shown to be resistant to DDT just four years following their use; see Sayono Sayono, Anggie Puspa Nur Hidayati, Sukmal Fahri, Didik Sumanto, Edi Dharmana, Surharyo Hadisaputro, Puji Budi Setia Asih and Din Syafruddin, “Distribution of Voltage-Gated Sodium Channel (Nav) Alleles Among the Aedes Aegypti Populations in Central Java Province and its Association with Resistance to Pyrethroid Insecticides,” PLoS One 11 (2016).
Kaliyaperumal Karunamoorthi and Shanmugavelu Sabesan, “Insecticide Resistance in Insect Vectors of Disease with Special Reference to Mosquitoes: A Potential Threat to Global Public Health,” Health Scope 2, no. 1 (2013): 4–18.
Heat also helps things get and stay moving: the rising temperatures that provide local indices to climate change also speed up Aedes Aegypti development and rapidly alter its behavior; every stage in their life cycle is condensed when incubated in heat, and warmer temperatures make Aedes Aegypti hungrier for blood.
Ibid., Sloterdijk, 28.
Saleha Sungkar, “Controlling the Dengue Vector: What’s the Problem?”, public lecture in the Faculty of Medicine, Universitas Indonesia (June 2016).
Judith Butler, Notes Toward a Performative Theory of Assemblage (Cambridge: Harvard University Press, 2015).
Ibid., Sloterdijk, 99.
Timothy Mitchell, Rule of Experts: Egypt, Techno-Politics, Modernity (Berkeley: University of California Press, 2002), especially 19–53.
Beatriz Colomina and Mark Wigley, Are We Human? Notes on an Archaeology of Design (Zurich: Lars Muller Publishers, 2016), 141.
Superhumanity is a project by e-flux Architecture at the 3rd Istanbul Design Biennial, produced in cooperation with the Istanbul Design Biennial, the National Museum of Modern and Contemporary Art, Korea, the Govett-Brewster Art Gallery, New Zealand, and the Ernst Schering Foundation.
Subject
The research which led to this publication was made possible by grants from the University of Pittsburgh and the NTU Centre for Contemporary Art Singapore. Specials thanks to the many researchers who helped conduct or supported this preliminary research, especially Christina Geros, Nashin Mahtani, Pritta Andrani, Mahardika Fadmastuti, Elise Hunchuck, and Frank Sedlar; thanks also to Richard Pell of the Center for PostNatural History for facilitating these and other postnatural encounters.